Number of pages: 100 | Report Format: PDF | Published date: May 24, 2023
Historical Years – 2021 | Base Year – 2022 | Forecasted Years – 2023 to 2031
|
Report Attribute |
Details |
|
Market Size Value in 2022 |
US$ 610.35 million |
|
Revenue Forecast in 2031 |
US$ 1418.62 million |
|
CAGR |
8.8% |
|
Base Year for Estimation |
2022 |
|
Forecast Period |
2023 to 2031 |
|
Historical Year |
2021 |
|
Segments Covered |
Product, Application, and Region |
|
Regional Scope |
North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa |
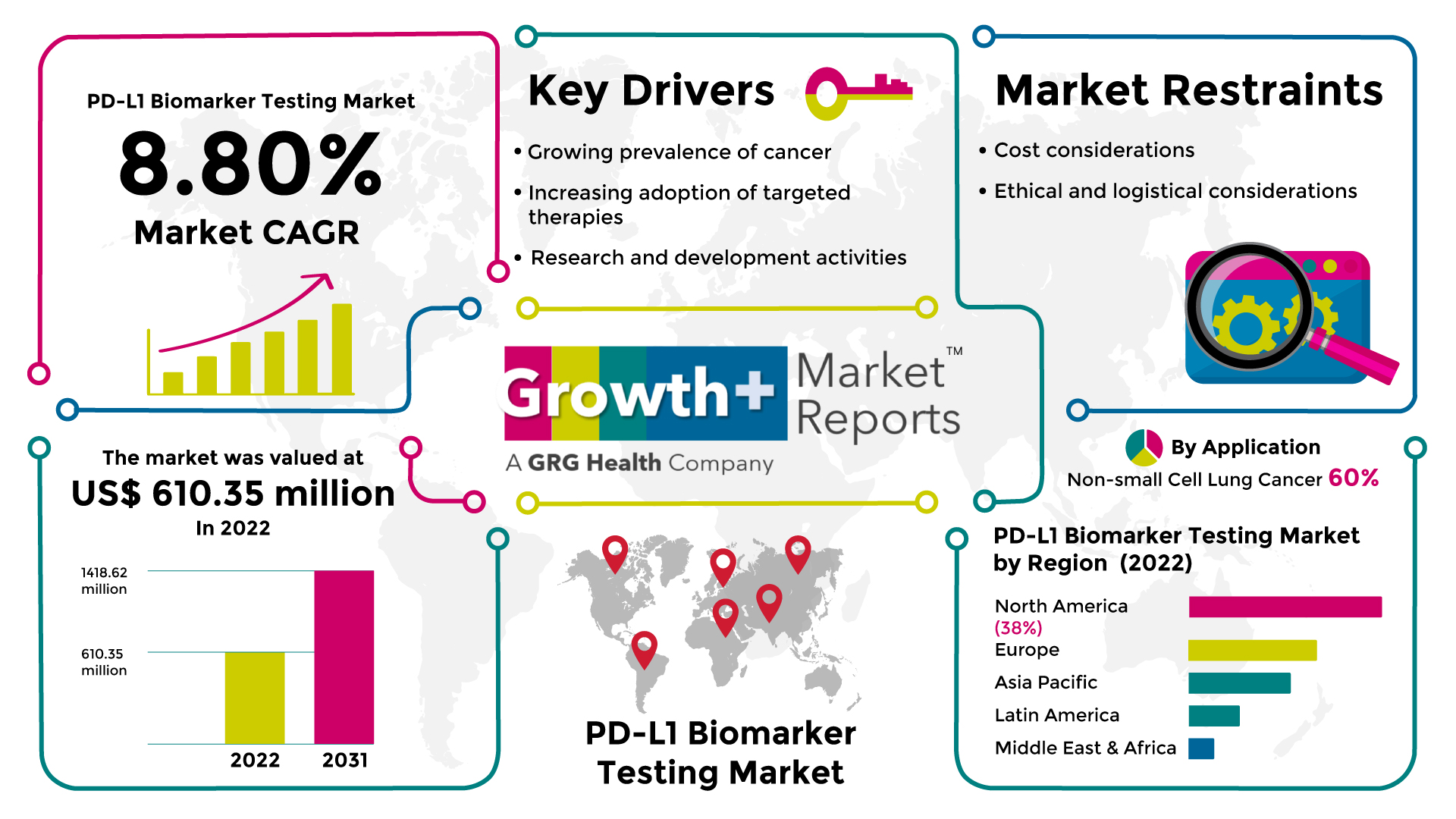
According to the deep-dive market assessment study by Growth Plus Reports, the global PD-L1 biomarker testing market was valued at US$ 610.35 million in 2022 and is expected to register a revenue CAGR of 8.8% to reach US$ 1418.62 million by 2031.
PD-L1 Biomarker Testing Market Fundamentals
PD-L1 biomarker testing refers to the process of evaluating the expression levels of programmed death-ligand 1 (PD-L1) protein in tumor cells or tissues. PD-L1 is a protein found on the surface of cancer cells that interacts with programmed death receptor 1 (PD-1) on immune cells, inhibiting the immune response against the tumor. Biomarker testing helps determine whether a patient's tumor has high or low levels of PD-L1, which can inform treatment decisions for certain types of cancer.
The global PD-L1 biomarker testing industry analysis report comprehensively analyzes the market, with the factors that either aid or impede the market growth, such as its drivers, restraints, and opportunities. The research provides insight into the companies that operate in the PD-L1 biomarker testing market and their efforts to position themselves as key players through expansion strategies and innovations. It also highlights recent developments that contribute to the PD-L1 biomarker testing market growth. The research also analyzes the impact of the COVID-19 pandemic.
This research is valuable for businesses seeking insights into the market, customers, and competition. The research provides special insights into the segmentation, regions, market size & projection, revenue CAGRs, and other important information that may help clients make the right decisions. The report assembles data from market players and professionals across the industrial value chain. The study also incorporates qualitative and quantitative assessments from industry professionals. Secondary research, surveys and interviews, and statistical modeling are all used in our research to estimate market size and projection. Our reports deliver the most reliable market data because of our specialized research methods.
PD-L1 Biomarker Testing Market Dynamics
The increasing cancer incidence worldwide is a significant driver for the PD-L1 biomarker testing market. PD-L1 testing is primarily used to manage various types of cancer, including lung cancer, melanoma, bladder cancer, and others. As the number of cancer cases rises, the demand for PD-L1 testing also increases. According to GLOBOCAN 2020 report, around 19 million new cancer cases were detected, and approximately 10 million deaths were cases by cancer. The emergence and success of immune checkpoint inhibitors, such as PD-1/PD-L1 inhibitors, have revolutionized cancer treatment. PD-L1 testing helps identify patients more likely to respond to these therapies. The expanding use of immunotherapy drives the demand for PD-L1 biomarker testing. PD-L1 testing plays a crucial role in determining the most appropriate treatment options for patients. It helps identify tumors more likely to respond to PD-1/PD-L1 inhibitors, allowing healthcare providers to make informed decisions about therapy selection. PD-L1 testing is often combined with other molecular tests to guide treatment decisions. As the use of targeted therapies continues to grow, including immunotherapies, the demand for PD-L1 testing as part of comprehensive molecular profiling increases. Ongoing research and clinical trials explore the role of PD-L1 expression in different cancer types and treatment settings. These studies generate evidence supporting the use of PD-L1 testing, driving market adoption as more data becomes available. Regulatory agencies have approved specific PD-L1 inhibitors and provided guidelines for their use. These approvals and guidelines often include recommendations for PD-L1 testing, driving PD-L1 biomarker testing market demand.
However, PD-L1 biomarker testing can be expensive, particularly when combined with other molecular tests. The high cost of testing may limit its accessibility, especially in resource-limited settings or regions with inadequate reimbursement coverage, which restricts the PD-L1 biomarker testing market. PD-L1 testing requires tumor tissue samples, which may not always be available or obtainable, especially in cases where repeat biopsies are not feasible or safe. Ethical considerations, patient preferences, and logistical challenges can affect the feasibility and practicality of conducting PD-L1 testing.
PD-L1 Biomarker Testing Market Ecosystem
PD-L1 Biomarker Testing Market, by Product
PD-L1 Biomarker Testing Market, by Application
PD-L1 Biomarker Testing Market by Application
The non-small cell lung cancer segment accounted for 60% of the market revenue share of the PD-L1 biomarker testing market. Lung cancer is one of the most prevalent types of cancer worldwide. According to the WHO, in 2020, around 2.21 million cases of lung cancer were diagnosed globally. The non-small cell lung cancer accounts for around 85% to 90% of lung cancer cases worldwide. This high prevalence contributes to a larger patient population needing biomarker testing, including PD-L1 testing, for treatment decision-making. Immunotherapy, particularly PD-1/PD-L1 inhibitors, has become a standard treatment option for advanced or metastatic non-small cell lung cancer. PD-L1 testing is widely used to identify patients more likely to benefit from these therapies, making it an integral part of the treatment selection process. PD-L1 testing has been extensively studied and validated in non-small cell lung cancer. Numerous clinical trials have demonstrated the predictive value of PD-L1 expression in determining the response to immunotherapy in non-small cell lung cancer patients. The availability of robust evidence supports the widespread adoption of PD-L1 testing in non-small cell lung cancer. PD-L1 expression status in non-small cell lung cancer has been associated with response rates and survival outcomes in patients treated with PD-1/PD-L1 inhibitors. This clinical relevance and the potential for improved patient outcomes drive the use of PD-L1 testing in non-small cell lung cancer.
The market segmentation sections provide the PD-L1 biomarker testing market outlook regarding the demarcation of different consumer groups. Market segmentation is splitting an industry into subgroups depending on characteristics such as product, technology, services, end user, and other factors. Market segmentation data helps organizations understand the preferences and distinctive demands of different customer groups and implement targeted marketing strategies. This data additionally helps in identifying potential PD-L1 biomarker testing market demand opportunities. The lumbar spine offers more treatment options than other spine regions. In addition to PD-L1 biomarker testing, alternative procedures such as lumbar fusion and disc replacement are commonly performed for lumbar spinal disorders. The availability of multiple treatment modalities in the lumbar segment contributes to its growth in the PD-L1 biomarker testing market.
PD-L1 Biomarker Testing Market by Region
The North America region held around 38% revenue share of the PD-L1 biomarker testing market in 2022. North America has a significant burden of cancer, including lung cancer, melanoma, bladder cancer, and others, for which PD-L1 testing is relevant. The high prevalence of these cancers drives the demand for PD-L1 biomarker testing in the region. According to the WHO, in 2020, 2.2 million new cancer cases were diagnosed in U.S. North America has been at the forefront of adopting precision medicine approaches, which aim to tailor treatments based on individual patient characteristics. PD-L1 biomarker testing plays a crucial role in guiding the use of immunotherapy, aligning with the precision medicine paradigm. The region has been a hub for developing and clinically using immune checkpoint inhibitors, including PD-1/PD-L1 inhibitors. The availability and success of these therapies drive the demand for PD-L1 testing as a predictive biomarker to identify patients who are more likely to benefit from immunotherapy. North America benefits from a well-developed healthcare infrastructure, including advanced laboratory facilities and access to cutting-edge technologies. This facilitates the implementation and availability of PD-L1 testing in the region.
Based on the regions, the global PD-L1 biomarker testing market is segmented into:
The industry's regional segmentation provides insights into geographic pockets regarding PD-L1 biomarker testing industry trends, market size, share, and growth rate. This information helps organizations assess potential growth opportunities in new regional markets, understand competitive threats, and develop localized sales and expansion strategies. This section also offers deeper insights into the regional and country-level PD-L1 biomarker testing market overview.
Key Components of the Report
PD-L1 Biomarker Testing Market Competitive Landscape
The market competitive landscape analysis is performed by gathering and evaluating data about the key competitors, industry trends, and market dynamics. It involves collecting and analyzing data on factors such as products, pricing, geographic reach, customer demographics, marketing tactics, and recent developments. Competitive landscape analysis can help organizations identify present or prospective opportunities and risks in the market.
PD-L1 Biomarker Testing Market Strategic Developments
Reasons To Buy This Report
Key Strengths of Our Report
Target Audience to Benefit from this Report.
PD-L1 biomarker testing refers to the process of evaluating the expression levels of programmed death-ligand 1 (PD-L1) protein in tumor cells or tissues. PD-L1 is a protein found on the surface of cancer cells that interacts with programmed death receptor 1 (PD-1) on immune cells, inhibiting the immune response against the tumor.
Asia Pacific is the key growth region in the global market during the forecast period from 2023 to 2031.
The PD-L1 biomarker testing market is expected to register a revenue CAGR of 8.8% during the forecast period from 2023 to 2031.
PD-1/PD-L1 inhibitors are currently approved for multiple cancer types, but there are still opportunities for further expansion of indications. As clinical evidence accumulates, regulatory agencies may approve these therapies for additional cancer types, broadening the potential market for PD-L1 biomarker testing.
Companies collaborate and partner with pharmaceutical manufacturers, research institutions, and diagnostic laboratories to facilitate the development and validation of PD-L1 testing in clinical trials and research studies.
The PD-L1 biomarker testing market was valued at US$ 610.35 million in 2022
The estimated size of the global PD-L1 biomarker testing market in 2031 is US$ 1418.62 million.
The key players operating in the global PD-L1 biomarker testing market are Thermo Fisher Scientific Inc., Agilent Technologies, Inc., F. Hoffmann-La Roche Ltd., Abcam plc., and Shuwein Biotech Co. Ltd.
The non-small cell lung cancer segment has the highest PD-L1 biomarker testing market share.
*Insights on financial performance are subject to the availability of information in the public domain